The phaseout of chlorofluorocarbons (CFCS) has resulted in the use of hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) as environmentally acceptable alternative chemicals. Trifluoroacetic acid (TFA) has been identified as a degradation by-product of these compounds, and is largely deposited to the Earth's surface by precipitation (Wallington et al., 1994). The global average TFA concentration in rainwater for the year 2010 is estimated to be in the range of 8 X 10-9- 1 x 10-7 M (Ball and Wallington, 1993). Little is known about the ecological and environmental fate of TFA.
Inputs of TPA to natural ecosystems may be biodegraded by microbial processes, assimilated by vegetation, adsorbed by organic and/ or mineral soil, or transported with drainage Water. Microbial degradation of trace levels of FTA has been reported (Visscher et al., 1994), but the significance in the environment is uncertain (Likens et al., 1996).
Trifluoroacetic is transported from roots via the transpiration stream and can bioconcentrate in leaf tissues of plants (Rollins et al., 1989; Thompsen, 1994). There are reports of growth inhibition in some plants at TFA concentrations one to three orders of magnitude higher than anticipated in precipitation (Ingle, 1968; Thompsen, 1994). The sorption to soil particles may influence the transport, bioavailability and toxicity of TPA.
Trifluoroacetic acid is a highly acidic organic compound that predominantly occurs as an anion under environmental conditions. This class of organics is considered highly mobile and susceptible to teaching from soils. However, the adsorption of acidic organic compounds is enhanced under conditions of low soil pH and high organic matter content. Soils of variable charge, such as allophanic and oxidic minerals also are able to adsorb the dissociated form of organic acids. A previous study of three agricultural, nonacidic soils, were found to retain only 0.1-2.6 % of added TFA (van Dijk, 1991). Unfortunately, the study did not include any soils that are known to retain organic anions, particularly soils enriched with iron and aluminum (Ultisols, Oxisols) or organic matter (Histosols).
Moreover, the concentration of TFA used in this experiment was four orders of magnitude larger than values anticipated in the environment over the next 20 years. Soil retention of TFA may play an important role in assessments of the ecological effects of this substance. Thus a cross site study was initiated to investigate factors regulating TFA retention in soil.
The objectives of this soil retention study were to:
- Evaluate the sorption of TFA on a variety of soils at concentrations comparable to anticipated values
- Evaluate the soil characteristics and conditions that influence TFA retention
- Evaluate the potential accumulation of TFA in soil of terrestrial ecosystems.
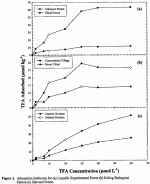
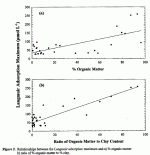
Thirty-five soil samples were obtained from 15 terrestrial sites of the National Science Foundation LTER program. A total of 54 soil samples from LTER sites and other locations were used in this study. Batch adsorption experiments showed that TFA was retained by 54 of 54 soils collected from diverse locations. No significant TPA retention was found in 20 of the soils studied. Retention ranged from a high of 260 µmol kg-1 to 25 µmol kg-1 (0-60 % of added TFA). In general, TFA was retained in organic soils to a greater extent than in mineral soils (Fig. 1). Organic soils that exhibited higher retention of TFA sorbed between 20-60% of added TPA. In contrast, mineral soils retained 0-15 % of added TFA. Organic soils with the highest TFA retention were from wetlands, peatlands, and a boreal forest. The magnitude of TFA adsorption in the majority of soils studied was small and similar to that of other monovalent anions (Cl and Br). Trifluoroacetate can be considered mobile in soils at the majority of sites investigated. Linear regression analysis between TPA adsorption and selected soil properties was used to identify soil constituents which influence retention and to better characterize the retention mechanisms. Trifluoroacetate retention exhibited a strong positive correlation with oxidizable organic matter content (r = 0.60; Fig. 2a). A strong relationship was also evident between TFA retention and the ratio of organic matter to clay content (r = 0.90; Fig. 2b).
Results from this study suggest that soil organic matter and clay surfaces influence the retention of' TFA by soils. There seem to be two mechanisms of TFA retention, indicated by differing shapes of TPA adsorption isotherms, for mineral (C-type) and organic (L-type) soils. Conformity of water-solub1e TFA to a Langmuir adsorption isotherm may indicate that the retention is site-specific. Further, the retention of TFA by soil particle surfaces is dependent upon pH and the presence of other anions in soil solution. Retention of TFA increased with decreasing soil suspension pH and decreased With increasing concentrations of competing anions. It is reasonable to assume that the factors regulating TFA retention by soils will also affect the transport of TFA in soil and drainage water. It is expected that differing leaching rates of TFA from soils will depend on soil types, soil organic matter content, soil pH, competing anion concentrations, and local rates of atmospheric deposition.
The ultimate fate of TPA in adsorbing soils is an important environmental question. Trifluoroacetate was significantly retained by wetland, peatland and boreal forest soils in this study. There is significant ecological importance associated with these ecosystems as they may be habitats for rare and endangered species (Shortelle et al., 1989). The retention of TPA by soils in these environments may be critical in assessments of local concentrations of TFA and the impact of the accumulation of TFA on these sensitive ecosystems.
In general, acidic solutes such as TFA, are weakly retained in soil. However, conformity to the Langmuir adsorption isotherm can indicate a stronger mechanism of retention. Adsorption isotherms cannot be interpreted to indicate any particular mechanism of retention, but this does indicate a need for further process level studies of TFA retention by soil organic matter. If TFA were not strongly adsorbed then the transport of TFA would be governed largely by the amount and frequency of precipitation and drainage. The strong retention of TFA may indicate that soil is a permanent sink for TFA. Because it is anticipated that soils will experience increasing TFA inputs over decades, the reversibility of TFA retention is critical for evaluating effects of TFA in terrestrial environments which exhibit soil retention.
For more information contact:
- Dorothy G. Richey (dgrichey@syr.edu)
- Charles T. Driscoll (cdriscoll@lternet.edu), Syracuse University
- Gene E. Likens, Institute of Ecosystem Studies, glikens@lternet.edu
Literature Cited
Ball, J.C., and Wallington, T.J. 1993. Formation of trifluoroacetic acid from the atmospheric degradation of hydrofluorocarbon 134a: A human health concern? Air and Waste, 43: 1260-1262.
Ingle, L.M. 1968. The response of wheat and tomato seedlings to seven halogenated aliphatic acids. Proc. West Virginia Acad. Sci., 40:1-11.
Likens, G.E., Tartowski, S.L., Berger, T.W., Richey, D.G., Driscoll, C.T., Frank, 1-I.C., and Klein, A. 1996. Transport and fate of trifluoroacetate in forest and Wetland ecosystems. Submitted to Nature.
Rollins, A., Barber, J., Elliott, R., and Wood, B. 1989. Xenobiotic monitoring in plants by 19F and 1H nuclear magnetic resonance imaging and spectroscopy: Uptake of trifluoroacetic acid in Lycopersicon esculentum. Plant Physiology, 91:1242- 1246.
Shortelle, A.B., Dudley, J.L., Prynoski, B., and Boyajian, M. 1989. American Water Resource: Association, 28:463-471.
Thompsen, R.S. 1994. AFEAS Workshop on the Environmental Fate of Trifluoroacetic Acid Ch. 15, F.G. Chumley, Ed. Miami Beach.
van Dijk, N.R.M. 1991. Adsorption test with sodium trifluoroacetate (NaTFA). Solvay Duphar, B.V. Internal Doc. No. 56635/9191.
Visscher, P.T., Culbertson, C.W., and Oremland, R.S. 1994. Degradation of trifluoroacetate in oxic and anoxic sediments: Rapid bacterial mineralization of an atmospheric hydrofluorocarbon degradation product. Nature, 369:729-731.
Wallington, T.J., Schneider, W.F., Worsnop, D.R., Nielsen, OJ., Sehested, J., Debruyn, WJ., and Shorter, J.A. 1994. The environmental impact of CFC replacements-HFCS and HCFCs. Environ. Sci. and Tech., 28 (7):320-326.

 Enlarge this image
Enlarge this image